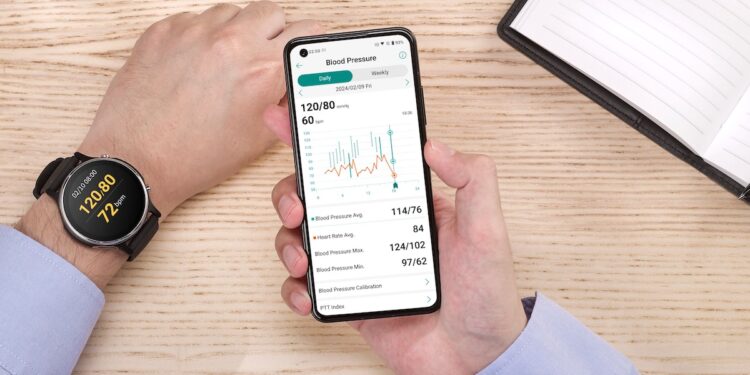
Food and Drug Administration of Thailand (FDA Thai) has approved their VivoWatch blood pressure app. This is an important step for ASUS in the health tech market in Southeast Asia. This is the first time the ASUS blood pressure app has received approval for medical use outside of Taiwan.
“We are happy to offer our new health solutions to people in Thailand,” said Joe Hsieh, Chief Operating Officer and Senior Vice President of ASUS. “This approval from the FDA Thai shows our commitment to creating trustworthy health technology that helps people manage their health.”
The ASUS blood pressure app is the first health wearable app approved by the Taiwan Food and Drug Administration. It works with supported ASUS VivoWatch models.
Users can record, store, and see their blood pressure readings, including systolic pressure, diastolic pressure, and heart rate. This feature will be available on ASUS VivoWatch 6, VivoWatch 5, VivoWatch 5 AERO, and VivoWatch SP for users in Thailand by the end of the year.
Also Read: Building Emotionally Intelligent Voicebots: The Next Frontier in AI Interaction